Search Results
Results for: 'molecule of ammonia'
Bond in biological molecules (Ionic, Covalent and Hydrogen bonds)/ How atoms bond?
By: HWC, Views: 8244
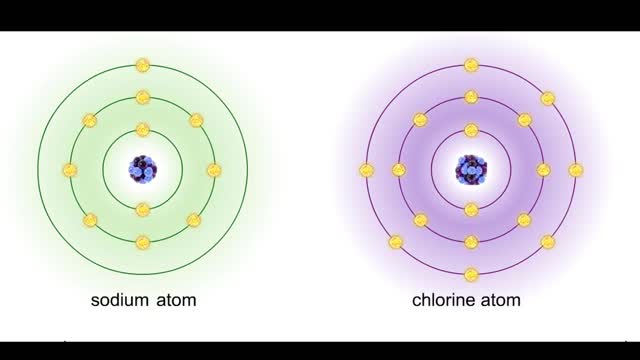
Sodium atoms and chloride atoms have unfilled orbitals in their outer shells. The lone electron in the outermost shell of a sodium atom can be pulled or knocked out. This ionizes the atom. It is now a positively charged sodium ion. A chlorine atom has an electron vacancy in its outer shell and...
By: HWC, Views: 9699
The slight positive charge of a hydrogen atom in a water molecule can attract an atom with a slight negative charge, such as the nitrogen in a molecule of ammonia. This forms a hydrogen bond between the two atoms. Hydrogen bonds join the two strands of a DNA molecule. Although hydrogen bo...
By: HWC, Views: 5118
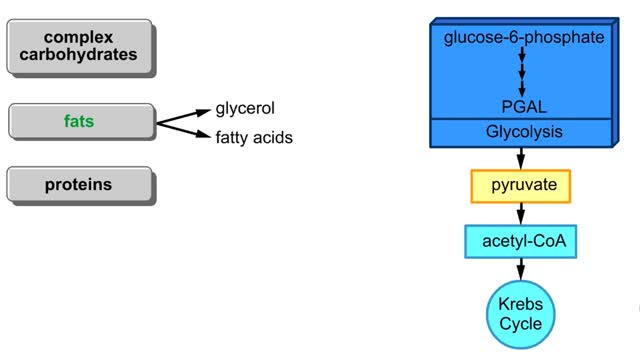
Points at which organic compounds enter the reaction stages of aerobic respiration. Complex carbohydrates are broken down into simple sugars, such as glucose. They become the substrates for glycolysis. If your body doesn't need to burn glucose for energy, glucose-6-phosphate can be co...
Structure of Amino Acid, Peptide Bonds & Polypeptides
By: HWC, Views: 10526
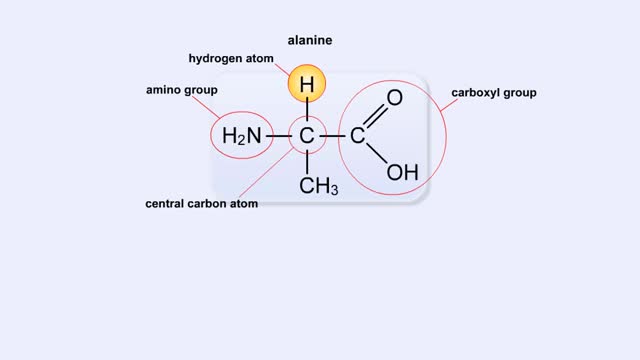
Here are the molecular formulas of three different amino acids. All amino acids share this backbone. The main difference between every amino acid is the side groups seen here, and these side groups give each of the amino acids their different characteristics. But before we get into that, let's ...
By: HWC, Views: 4906
In glycolysis, a six-carbon glucose molecule is split into two three-carbon pyruvate molecules. In this animation, each carbon molecule is represented by a red ball. The end products of glycolysis are two molecules of pyruvate. Glycolysis is the breakdown of glucose into two molecules of ...
Miller's reaction chamber experiment Animation
By: HWC, Views: 4597
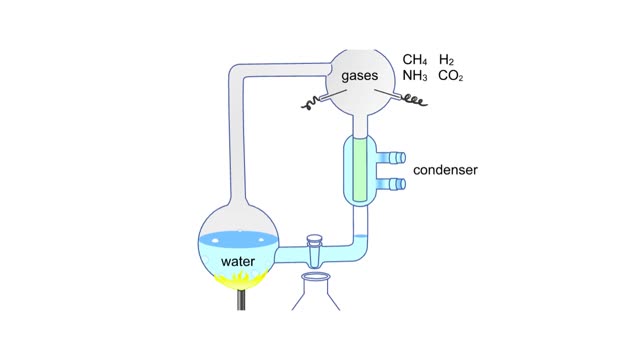
A simple diagram of Stanley Miller and Harold Urey's experimental apparatus. The lower portion of the apparatus was filled with water. The upper portion was filled with a mixture of gases that simulated the earth's early atmosphere. Examples are methane, ammonia, hydrogen and carbon dioxide. ...
Splitting of Sugar, Oxidation/ Reduction & ATP Generation
By: HWC, Views: 10660
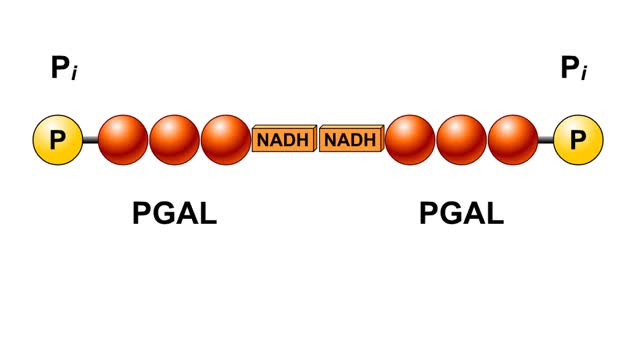
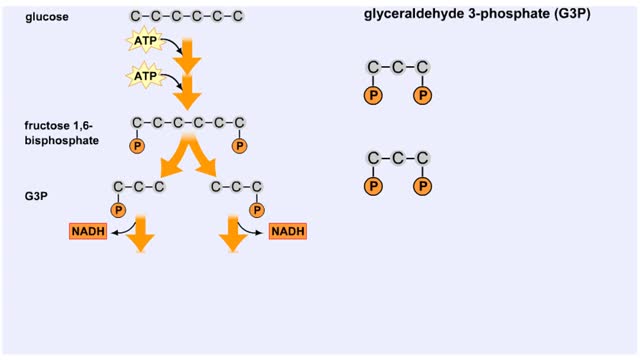
The next reaction shows us the meaning of "glycolysis" or the splitting of glucose. The fructose bisphosphate molecule is split into two molecules each containing 3 carbons as the backbone. FBP is split into two 3-carbon molecules called G3P, or glyceraldehyde 3-phosphate. Notice that the phos...
Condensation and Hydrolysis Animation
By: HWC, Views: 4727
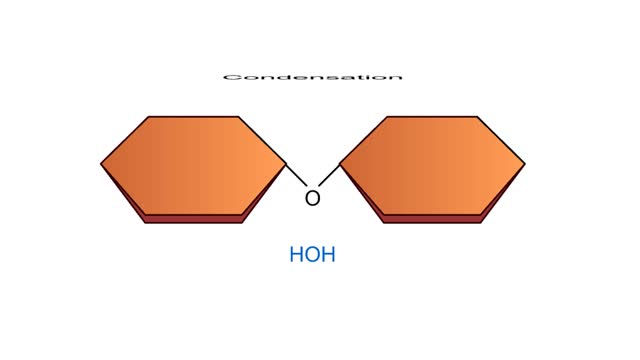
A condensation reaction joins two molecules together to form one larger molecule. An enzyme removes a hydroxyl group from one molecule and a hydrogen atom from another, then speeds the formation of a bond between the two molecules at their exposed sites. Typically the discarded atoms join t...
Calvin cycle (The light-independent reactions )
By: HWC, Views: 10711
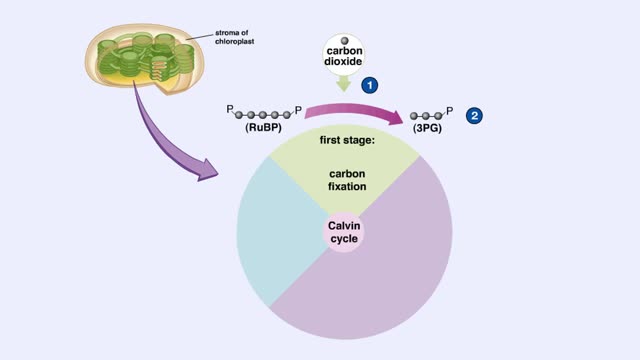
The light-independent reactions of photosynthesis occur in the stroma of the chloroplast. Carbon dioxide enters the leaf through tiny pores or stomata and diffuses into the chloroplast. The first stage of the Calvin cycle is the attachment of a carbon dioxide molecule to a 5-carbon ribulose bi...
Advertisement